The science behind Water-King
The Principle
The ability of electro-magnetic induction waves or radio waves to alter the crystal structure of salts dissolved in water is the subject of considerable debate among scientists.
Aside from many papers published recently on the subject, a one-day seminar was held at The Department of Water Sciences, Cranfield University, Bedford, in March 1996, at which some of the world's foremost scientists spoke on the effect of various magnetic fields on crystal shapes and in particular the shape of calcium crystals. A two page synopsis of the Cranfield seminar is available free of charge from Advanced Environmental. A comprehensive science pack, including bibliography and full reports, is also available at a cost of $12
What exactly is 'Hard' Water?
Normal rain and acid rain (partly caused by the dissolved gases of Sulphur Dioxide and Carbon Dioxide) leach out calcium salts from the ground. The commonest of these is Calcium Carbonate, which dissolves easily. The result is calcium rich water, which we all call 'hard'. When such water is heated, or its pressure reduced, the Calcium Carbonate precipitates and forms the limescale which causes so many problems.
The Technology of Water-King
Specially pre-programmed micro-chips in Water-King units control the broadcast asynchronously of 350+ varying low-frequency electro-magnetic radio waves. They are transmitted into the hard water by two or more aerials wrapped around the water supply.
These continually varying low-frequency radio waves, which are totally harmless, cause the calcium salts that produce limescale to assume a different crystal structure. The change is achieved by a de-stabilisation of the Ca++ ions of Calcium and the CO3-- Carbonate ions, which then combine, when the temperature rises, to form a crystal structure called Aragonite (Orthorhombic CaCO3). Without the low-frequency electro-magnetic radio waves, the same ions would combine to form a solid mixture of Calcite and Aragonite Crystals which, together with another crystal called Vaterite, creates limescale.
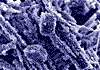
1. Limescale - amorphous
crystals x 50 |

2. Pure Calcite crystals* |

3. Limescale in water
cylinder |

4. Pure Aragonite
cyrstals x50 |
* Courtesy of Oxford University Museum
Pure Calcite (Rhombohedral CaCO3) takes the form of a rhombic crystal which adheres very strongly and can form Marble. Aragonite, on the other hand, is soft, non-adhesive, stays in suspension and is easily flushed away.
Existing limescale deposits also change to Aragonite and just dissolve away. The Cranfield Report (see above) clearly indicates that the shape of the final Calcium Carbonate crystal is determined at the point of nucleation or crystal seeding and that this event is affected (and the shape pre-determined) by the energy imparted to it at the time.
The Water-King unit can cause a change in the treated water for up to five days (Water Memory). The variable frequencies not only change the morphology of Calcium Carbonate, but also prevent immunity from developing.
The treated water becomes "synthetically" soft and, whilst it retains all the valuable minerals such as calcium, it behaves in nearly every respect like chemically treated soft water. It is believed that changes at the surface of the water molecule may explain this condition (see Cranfield Report).
Hard Water || Other Solutions? || The science behind || Q and A || Free Tester Kit

|
